Litium‑ion Batteries
What is a Lithium-ion battery and how does it work?
The most common electrical storage device is the battery. Primary batteries can be used once and have electrode materials that are irreversibly changed during discharge. Secondary batteries, or rechargeable batteries, can be charged and discharged multiple times and have electrode material that can be restored by reversing the current. A battery stores energy electrochemically; that is, chemical compounds are formed at the interface between the electrode and the electrolyte, releasing electrons during discharge. During charging, electrons move in the opposite direction and the chemical reaction reverses.
Lithium-ion batteries (LIBs) currently have the highest gravimetric and volumetric energy densities and are the battery of choice for most portable electronics, military applications and for electric vehicles.
Lithium is the lightest metal, has the greatest electrochemical potential, and thus provides the largest specific energy per unit mass if used in batteries. LIBs with pure lithium as anode could theoretically provide an extraordinary high energy density, however, charging and discharging causes problematic dendrites on the anode that could penetrate the separator and cause a short circuit. This instability shifted research from lithium metal to a non-metallic solution using lithium ions. Although lithium ions provide lower specific energy than lithium metal, they are safer and outperform all other rechargeable battery chemistries with respect to energy and power density, and currently, this chemistry has become the most promising and fastest growing on the market. Meanwhile, research continues to focus on developing a safe metallic lithium battery.
LIBs, as well as all electrochemical cells, comprises two electrodes, the anode and the cathode separated by an electrolyte. The cathode is a metal oxide and the anode usually consists of graphite. The electrolyte can either be solid or liquid but solid electrolytes are still quite rare due to the complex fabrication of solid–solid interfaces with good ion-permeability. One exception is the use of solid polymer electrolytes and thin electrodes. Electrodes separated by a liquid electrolyte are held apart by an ion-permeable separator.
During charge, the lithium ions move from the cathode (positive electrode), through the electrolyte and separator, to the anode (negative electrode) and intercalates into the electrode material. If graphite is used as anode, intercalation simply means that the lithium ions get inserted between the graphene layers in the graphite structure. During discharge, the lithium ions move back in the opposite direction, restoring the lithium compound in the cathode and reach a lower net energy state. Simultaneously, the electrons flow through the external circuit in the same direction.
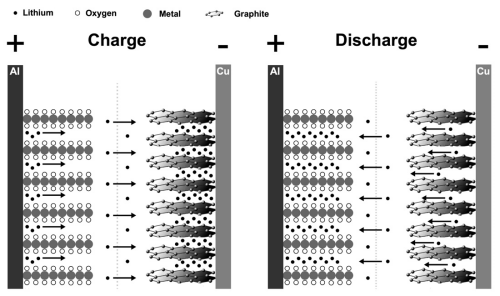
Figure: Schematic sketch of the simplified energy storage principle in a Lithium-ion battery, during charge and discharge.
LIBs comes with many different cell chemistries, with a rather extensive range of energy density, power density, and safety. The LIBs are often named after the metal oxide used as the cathode material. Lithium cobalt oxide (LCO), introduced in 1991, was the first commercial available cathode material and has since then been used for both consumer electronics and EVs (early Tesla Roadster). Among the LIBs on the market today, Lithium Iron Phosphate (LFP), Lithium Manganese Oxide (LMO), Lithium Nickel Manganese Cobalt Oxide (NMC), and Lithium Nickel Cobalt Aluminum Oxide (NCA) are available. These cathode materials are mainly used with a graphite anode except a few manufacturers that use graphite and silicon or lithium titanate to improve the storage capacity or power capability and cycle life. Some manufacturers also mix the cathode material to reach desired performance.
Challenges / Possibilities
LIBs have been using graphite anodes since the introduction of LCO in 1991, and graphite or carbon continues to be the anode material of choice for most commercial LIBs. For high power applications, some manufacturers use lithium titanium oxide anodes for improved power density and cyclability but at the expense of lower energy density and higher material cost. New developments on the field of LIB anodes has garnered considerable large attention toward silicon due to its extremely high theoretical storage capacity and low cost. Silicon can facilitate up to 4.4 lithium ions per atom, compared to one lithium ion per six carbon atoms, which would significantly increase the cell energy density. However, the large volume change of silicon when lithium gets inserted (more than 300 %) is a major obstacle as it causes material cracking, and currently, only small amounts of silicon are used with carbon. One way of solving this material crumbling issue seems to be the use of nanosized silicon particles embedded in a matrix of graphene, graphite or conductive polymers.
Our research
Silicon-nanographite anode composites
Read more
