Supercapacitors
What is a Supercapacitor and how does it work?
Conventional battery technologies possess large energy densities but still rather poor power densities and are more suitable for applications requiring large amounts of energy or energy stored for a longer time. Supercapacitors possess the opposite characteristics with poor energy storage densities but excellent power densities. New developments in advanced materials and hybrid solutions have shifted both conventional batteries and aupercapacitors toward the upper right corner of the Ragone plot. Lithium-ion batteries have taken a major step towards both higher energy and power density, however, with regard to high power cyclability, supercapacitors still have the greater advantage.
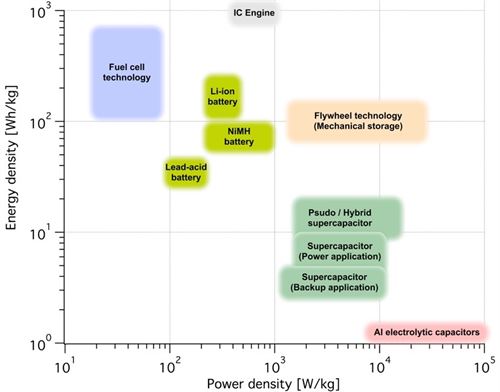
Figure 1: Ragone plot comparing capacitors and batteries. For further comparison the Ragone plot also contain fuel cell technology and internal combustion engines, even though these are not energy storage devices.
Supercapacitors and ultracapacitors are both alternative names for a class of electrochemical energy storage devices. Initially, supercapacitors was an alternative name for electrical double-layer capacitors (EDLC), but presently, the name supercapacitors usually also includes pseudocapacitors and hybrids.
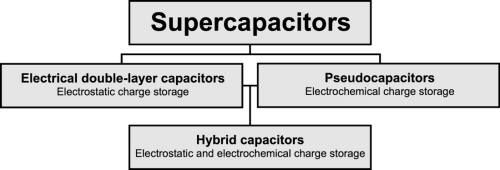
Figure 2: The hierarchical classification of supercapacitors.
Each supercapacitor cell comprises two electrodes separated by a porous ion-conductive insulator. Each electrode is connected to a current collector and the entire cell is soaked in the electrolyte. EDLC only facilitates charge separation and does not undergo any electrochemical reactions, resulting in relatively poor energy storage capacity but a very high power density and superior cyclability, with a lifetime of approximately 1,000,000 charge/discharge cycles. EDLCs can store and release electrical energy by nanometer-scale charge separation. The charge separation occurs rapidly at the interface between a porous electrode and an electrolyte. Pseudocapactitors facilitate electrochemical charge storage (similar to batteries) by Faradaic electron charge-transfer with e.g. redox reactions or intercalation. Hybrid capacitors are a mix of EDLCs and pseudocapacitors and often use two electrodes with different characteristics, one for electrostatic charge storage and one for electrochemichal charge storage. Pseudo- and hybrid capacitors have higher energy density than EDLCs due to their electrochemical charge storage and are thus approaching the energy density of batteries. The disadvantage, however, is that Pseudo- and hybrid capacitors, at the same time, sacrifice cyclability, lifetime, and pulse power efficiency.
Double layer principle
When a voltage is applied to the supercapacitor, an electric double-layer is formed at the interface between the electrode and the electrolyte, separating the electrolyte ions into a mirror charge distribution of opposite polarity. Such structures are referred to with different names depending on the model used to describe the double-layer. The developed Stern model, referred to as the Bockris-Devanathan-Müller (BDM) model, is often used; this model is based on a combination of the Helmholtz model and the Gouy-Chapman model.
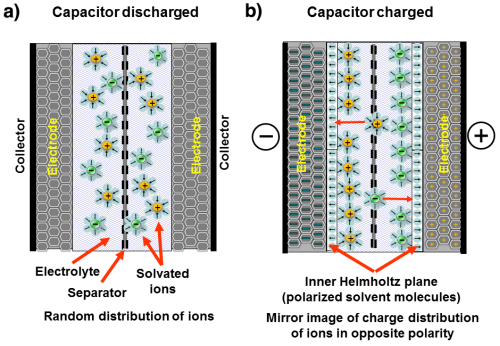
The figure shows a simplified schematic sketch of the cell structure and the function of an ideal supercapacitor. a) corresponds to a discharged supercapacitor where the ions are randomly distributed in the electrolyte, and b) shows a charged supercapacitor where the ions are separated by creating a charge distribution.
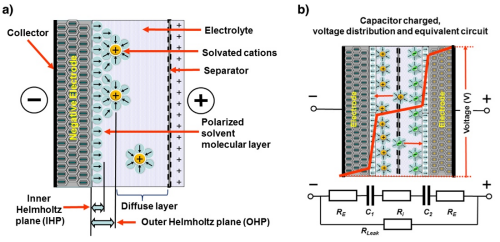
The figure shows (a), a simplified sketch of the Helmholtz planes in the supercapacitor and (b), the voltage distribution inside the supercapacitor and a simplified DC circuit.
The Helmholtz double-layer comprises an electronic layer on the surface of the electrode and another layer with opposite polarity from the dissolved ions in the electrolyte. These two layers are separated by a monolayer of solvent molecules called the inner Helmholtz plane. The solvent molecules adhere to the electrode surface by physical adsorption and separate the oppositely polarized ions from each other, serving as a dielectric layer of a single molecule's thickness. No charge transfer occurs between the electrode and the electrolyte so the adsorbed molecules do not undergo chemical changes.
The electrolyte charge layer forms the outer Helmholtz plane, and the charge capacity in the electrode is matched by the counter-charges in this plane. The double-layer capacitance, Cdl, consists of two parts, the capacitance from the Helmholtz planes and the diffuse layer capacitance. To estimate Cdl in a more simplified approach, a modified equation of parallel plate capacitance can be used.
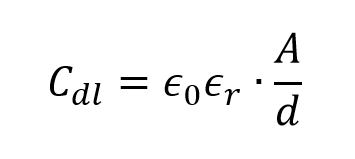
where e0 is the permittivity of free space, er is the relative permittivity of the electrolyte medium, A is the surface area of the electrode and d is the distance of charge separation, in this case the very small distance between the electrode surface and the outer Helmholtz plane. Figure 1.3 shows that the supercapacitor cell consists of two capacitors, C1 and C2, in series. It can be observed that a large electrode surface area together with a nanometer-scale charge separation distance gives rise to a very large capacitance for supercapacitor compared with a regular parallel plate capacitor.
Electrode material
Carbon is one of the most abundantly accessible and structurally varied materials on the planet. Many different carbon structures can be used in supercapacitor electrodes each with the same target to obtain an electrode with good electrical conductivity and high surface area. Conventional supercapacitors generally have an electrode material that is primarily made of highly porous activated carbons mixed with a conductive additive, e.g., carbon black and a small amount of binder. Alternatively, highly porous activated carbons can be mixed directly with a conductive binder. The activated carbons are porous, with numerous micro-, meso-, and macro-pores, and the surface area can be in the order of 1000 m2g-1, resulting in measured capacitances of 100 Fg-1 or more. The difference between micro, meso, and macro pores in activated carbons is in the pore size: microporous carbons have pore diameters of less than 2 nm, mesoporous carbons have pore diameters from 2 nm to 50 nm, and macroporous carbons have pore diameters larger than 50 nm. The disadvantage of high porosity is poor conductivity, resulting in the need for conductive additives or binders with good conductivity. The properties of activated carbons differs depending on its structure and the distribution of pore size. In carbons with large pores, the solvated ions form an interface layer close to the surface, while the remaining pore volume is filled with the electrolyte solvent. If the pores are smaller than the ions, the ions are blocked and the pore volume does not contribute to the charge separation character. The optimal pore size, with respect to the storage density, is achieved when the pore width is close to the ion diameter, forcing the ion to desolvate when entering the pore; this process reduces the charge distance and enhances the capacitance. Activated carbon can be prepared from a variety of sources with different properties, from natural precursors to petroleum residues and synthetic tailor-made nanostructures. The most common alternative used for commercial supercapacitors is coconut-based activated carbon, which offers a compromise in purity, conductivity, surface area, and price.
Binder
In addition to the active carbon material, the binder is a significant component of the electrode. The binder has two functions: it enables adhesion to the current collector, and creates strong cohesion between the electrode particles. The type and amount of the binder are adjusted to ensure the following: electrolyte impregnation of the electrode particles without intergranular blocking and maximum particle-particle and particle-collector contact with minimal electrical resistance. The most commonly used binders are insulating polymers, vinyl or cellulosic alternatives such as polytetrafluoroethylene (PTFE), polyvinyl alcohol (PVA), and carboxy-methyl-cellulose (CMC), with PTFE being the most common. Recently, cellulose nanofibrills (CNF) also referred to as nanofibrillated cellulose (NFC) has been used as a binder in carbon structures with promising results.
Current collector
Current collectors are used to transport the electrical charges between the active electrode material and the external connection terminals of the supercapacitor unit. The current collector needs to exhibit low electrical resistivity, low interfacial resistance toward the electrode, and electrochemical stability in the electrolyte. The cost and processability of the current collector are also important parameters for large-scale use. Owing to its high conductivity, low weight, and favorable price, aluminum foil is the most commonly used current collector in commercial supercapacitors in combination with organic electrolytes. For supercapacitors with aqueous electrolyte, the demand for electrochemical stability increases. The aggressive nature of aqueous electrolytes, often based on strong acids or bases, puts a high corrosion stress on the current collector and prevents the use of aluminum. Even in neutral pH, the corrosion potential (the potential for galvanic corrosion) between aluminum and graphitic carbon is high enough to cause pitting in the aluminum foil.
Electrolyte
Electrolytes, of which various types exist, each with different characteristics, are crucial to the function and performance of a supercapacitor. Electrolytes can be both solid and in solution; however, the most common types are aqueous electrolytes, organic electrolytes, and ionic liquids (molten salts). Aqueous electrolytes, such as 1 M sodium sulfate, sulfuric acid, or potassium hydroxide have an electrochemical stability window of 1.23 V in water. At higher potentials, the water begins to decompose into oxygen and hydrogen. This window is wider for organic electrolytes operating at 2.2-2.7 V and is significantly wider for ionic liquids operating at 5 V. The electrochemical stability window substantially affects the storage capacity of the device as the energy stored in a supercapacitor is proportional to the square of the applied voltage.
Most commercial supercapacitors use organic electrolytes such as 1 M tetra-ethyl-ammonium in acetonitrile or propylene carbonate. The primary advantage of organic electrolytes over ionic liquids is their fairly wide electrochemical stability window, their compatibility with aluminum current collectors, and their high conductivity. The major drawbacks are that organic electrolytes are flammable and in some cases toxic.
Separator
The separator is a passive component in the supercapacitor which prevents contact and electron transfer between the two electrodes. It is necessary for the separator to be a good electrical insulator, strong enough to prevent electrode particle migration, and a good ion conductor to allow the electrolyte ions to diffuse freely through the separator. The material used in commercial separator films varies depending on the choice of electrode material, the electrolyte, and operating temperature range. The separators used are often the same as those for batteries and chiefly comprises micro-porous polymers, but cellulose papers, glass, mica, and ceramics are also used.
Challenges / Possibilities
Electrode material
In new developments, a variety of nanostructured carbons have been tested with promising results. Nanostructured carbons such as carbon nanotubes and graphene exhibit superior electrical conductivity, and the specific surface area of graphene is more than 2600 m2g-1, resulting in a theoretical capacitance of up to 550 Fg-1. Measured capacitance values greater than 200 Fg-1 and charge current densities of 100 Ag-1 have been reported for supercapacitors with nanostructured carbon electrodes making these materials promising, but yet too expensive for automotive and grid applications.
Current collector
To prevent the corrosion of the current collector in supercapacitors with aqueous electrolytes, well-known materials with high corrosion resistance such as stainless steel, titanium, nickel and platinum are used. However, these materials are significantly more expensive than aluminum and are also heavier and usually more difficult to process to achieve low interfacial resistance.
Electrolyte
Although aqueous electrolytes have the most narrow electrochemical stability window and an aggressive influence on the current collectors, their favorable cost and environment-friendly aspects are promising. They are less expensive, nonflammable, have higher ionic conductivity, and yield higher capacitance due to smaller ions.
